- Ischemic Stroke, Inflammation, Autonomic Nervous System, Heart-Brain Axis, Microbiota, Microbiome
The association between Inflammation and Pre- and Post-Ischemic Stroke.

Author: Dr. Andréa Fuzimoto
Disclosure: This site contains links to our store. Some others may be affiliate links by which we may earn a small commission when you purchase certain products or services. This does not affect the prices that you may pay for them. By purchasing from us you help support this website and our services. Thank you!
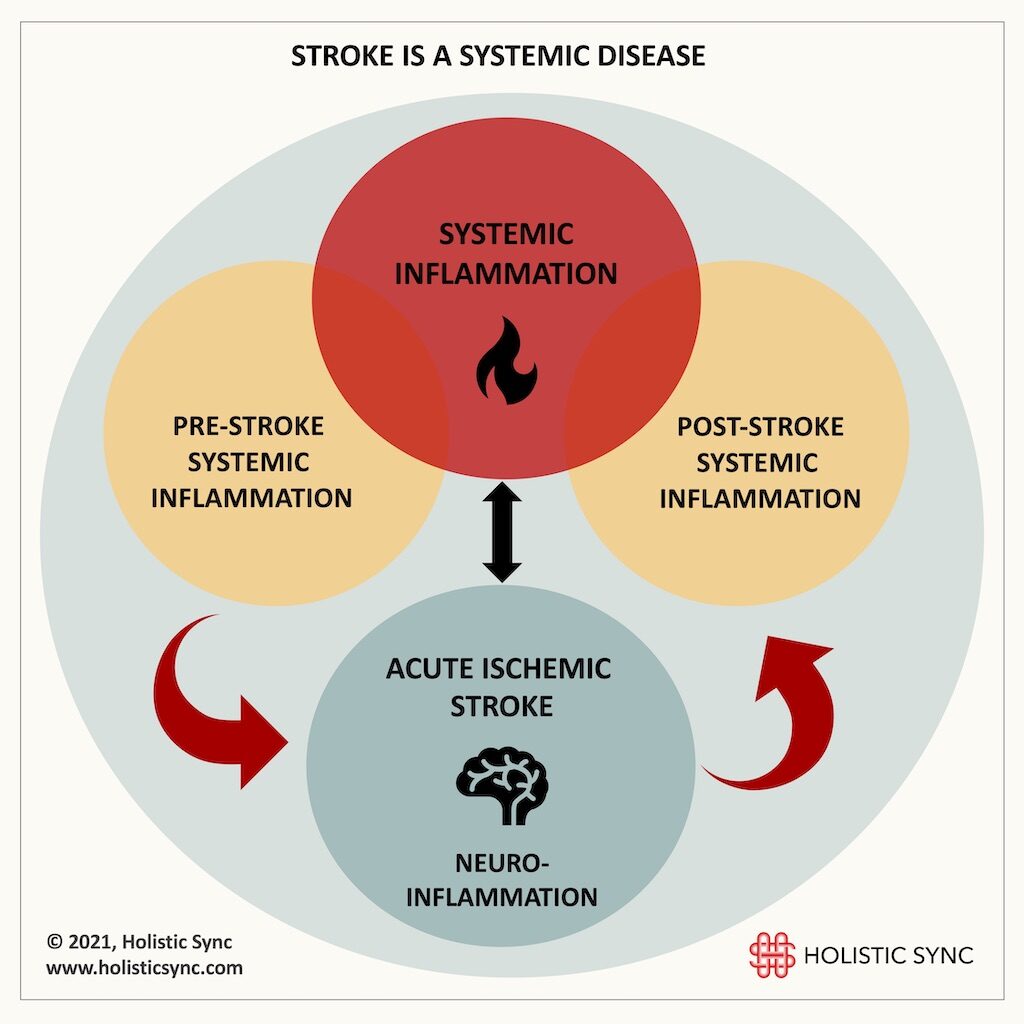
Stroke has shifted from brain disease to a systemic condition with the involvement of multiple signaling pathways of the neuro-endocrine-immune system. Pre-stroke systemic inflammation due to chronic conditions, neuroinflammation generated by the brain injury itself, and post-stroke systemic inflammatory responses are related factors that reflect a complex disease pathogenesis.
Diseases that cause chronic low-grade systemic inflammation such as hypertension, atherosclerosis, coronary artery disease, diabetes, obesity, and smoking predispose people to strokes. In turn, when a stroke happens, it causes brain and systemic inflammation.1 The pre-stroke systemic inflammation persists after the acute ischemic stroke and likely influences the disease recovery and outcome.1 The pre- and post-inflammatory processes create a positive feedback loop that reinforces the disease pathogenic processes. The persistence of chronic neuroinflammation after a stroke is linked to many complications such as depression, fatigue, and cognitive impairment. By the time a person experiences a stroke, the health scenario can be very complex involving the brain and multiple organ systems. With the last years’ research, today stroke is considered not an exclusive brain disease but a multifactorial and systemic condition with important consequences and sequelae.2 Thus, stroke treatment strategies need to consider the brain as well as other systems outside it.
Post-stroke neuro-immune responses
Ischemic strokes constitute 80% of all stroke types and it is caused by a blockage of an artery that provides blood circulation to the brain. The occlusion can be caused by different factors such as a blood clot or emboli. When a stroke happens, a focal area of the brain is deprived of oxygen and glucose which causes damage to that part of the brain, leading to neuron death. Around the ischemic core, a surrounding area called “penumbra” is also injured but cell death happens at a slower pace and may be salvaged.3 Right after the stroke, the immune system reacts locally sending immune cells and proteins to contain and repair the damage, and remove dead cells. Not only neurons are damaged but also other types of cells such as glial and endothelial cells. After the stroke, pro-inflammatory mediators increase while anti-inflammatory cytokines decrease.3 Besides the brain neuroinflammation, patients experience excitotoxicity, neurotoxicity, oxidative and nitrative stress, mitochondrial failure, and ATP reduced levels, to cite a few changes. However, another series of neuro-immune events outside the scope of the local injury happens before and after the stroke:
- Systemic Inflammation: Systemic inflammation activates the innate immune response of the central nervous system, alters neurotransmitter function, increases already pre-existing inflammatory processes in the brain, and exacerbates symptoms of neurodegenerative diseases (e.g. multiple sclerosis, Parkinson’s disease, Alzheimer’s disease, or prion disease).1 Studies using ischemic stroke models showed that systemic inflammation aggravates the ischemic damage, the severity of neurological deficit, and brain edema.1 Systemic inflammation disrupts the blood-brain-barrier (BBB), a barrier that prevents certain molecules and unwanted substances from crossing into the brain. With the increase in permeability of the BBB, peripheral inflammatory cytokines (IL-6 and C-reactive protein) and other cells such as monocytes and neutrophils that are produced during the systemic inflammation can cross into the brain and trigger or increase neuroinflammation.1,2 Also, one-third of the ischemic strokes are triggered by Infections which in many cases may also generate chronic systemic inflammation.1 Thus, evidence demonstrates that besides predisposing to stroke, systemic inflammation worsens brain inflammation.
- Autonomic Nervous System (ANS): The ANS is composed of the Sympathetic and Parasympathetic Nervous Systems (SNS and PNS) and regulates a series of functions in the body. The SNS is responsible for the “fight or flight” response to stress and the PNS activates the state of “rest and digest”. Overall, the SNS supports inflammation while the PNS promotes anti-inflammatory activity. Some people develop a chronic sympathetic overactivation thus making them more predisposed to inflammation. ANS dysfunction, especially with hyperactivation of the SNS and hypoactivation of the PNS is linked to cerebrovascular diseases.4 Theories state that ANS dysfunction increases the risk of stroke and that post-stroke ANS dysfunction increases mortality and morbidity.4 In fact, several diseases that predispose to strokes such as hypertension and obesity have altered ANS function with increased sympathetic response.4 Since the ANS regulates different organs such as the heart, blood vessels, kidneys, liver, intestines, urinary bladder, lungs, digestive glands, stomach, and others, a post-stroke ANS dysfunction will impact several systems.4
The Autonomic Nervous System and Stroke
2.1. Sympathetic nervous system (SNS): After a stroke, there is an increase in catecholamine levels such as noradrenaline (NE) and activation of the SNS. The increased sympathetic response during and right after the acute stroke causes an inflammatory response. However, later when the spleen depletes its reserves of immune cells, there is systemic immunosuppression of T, B, and natural killer (NK) cells which predisposes the person to post-stroke infections (e.g. pneumonia and urinary tract infections).2,4,5 Not only the spleen but also the liver are involved in the post-stroke immunosuppression as a response to sympathetic hyperactivation.4
2.2. Parasympathetic nervous system (PNS): During the post-stroke period there is also a decrease of the PNS activity. An increase in PNS response is considered protective in ischemic strokes and works in different ways to benefit the patient. For example, while the sympathetic nervous system contributes to the vasoconstriction of arteries, a parasympathetic activation will cause vasodilation of arteries and an increase in blood flow in the ischemic area thus limiting the brain damage.4 A sympathetic-parasympathetic balance is imperative for cerebral blood flow.
A hyperactive sympathetic and hypoactive parasympathetic response are linked to impaired ischemic stroke recovery.4 Although the SNS/PNS are involved before and after acute stroke, there are still controversies in how they could be used therapeutically to help in stroke recovery. However, studies show that this unbalanced ANS can remain for a long time after stroke, and addressing it may help patients recover faster, more thoroughly, and avoid other strokes.
Strokes and the heart-brain connection
Another important factor is that ischemic stroke is harmful to the heart, and at the same time heart disease is a major risk for stroke. One mechanism involved in the interrelationship between the heart and the brain is also related to the autonomic nervous system (ANS). After the stroke, the ANS is dysregulated (SNS/PNS), and the catecholamines norepinephrine (NE) and epinephrine (E) are increased.4 The heart has receptors that respond to these catecholamines causing a series of events such as an increase in intracellular calcium and oxidative stress leading to damage of cardiac muscle cells (myocytes).4 NE/E also affects the blood vessels causing them to constrict thus decreasing the cardiac blood flow.4 Additionally, arrhythmias and hypertension are common after a stroke due to the increased sympathetic response. However, in most patients, the blood pressure may normalize a few days after the stroke. Treatment strategies for stroke must include management of pre-existing heart conditions as well as post-stroke cardiac dysfunctions.4
Strokes and gut microbiota-microbiome
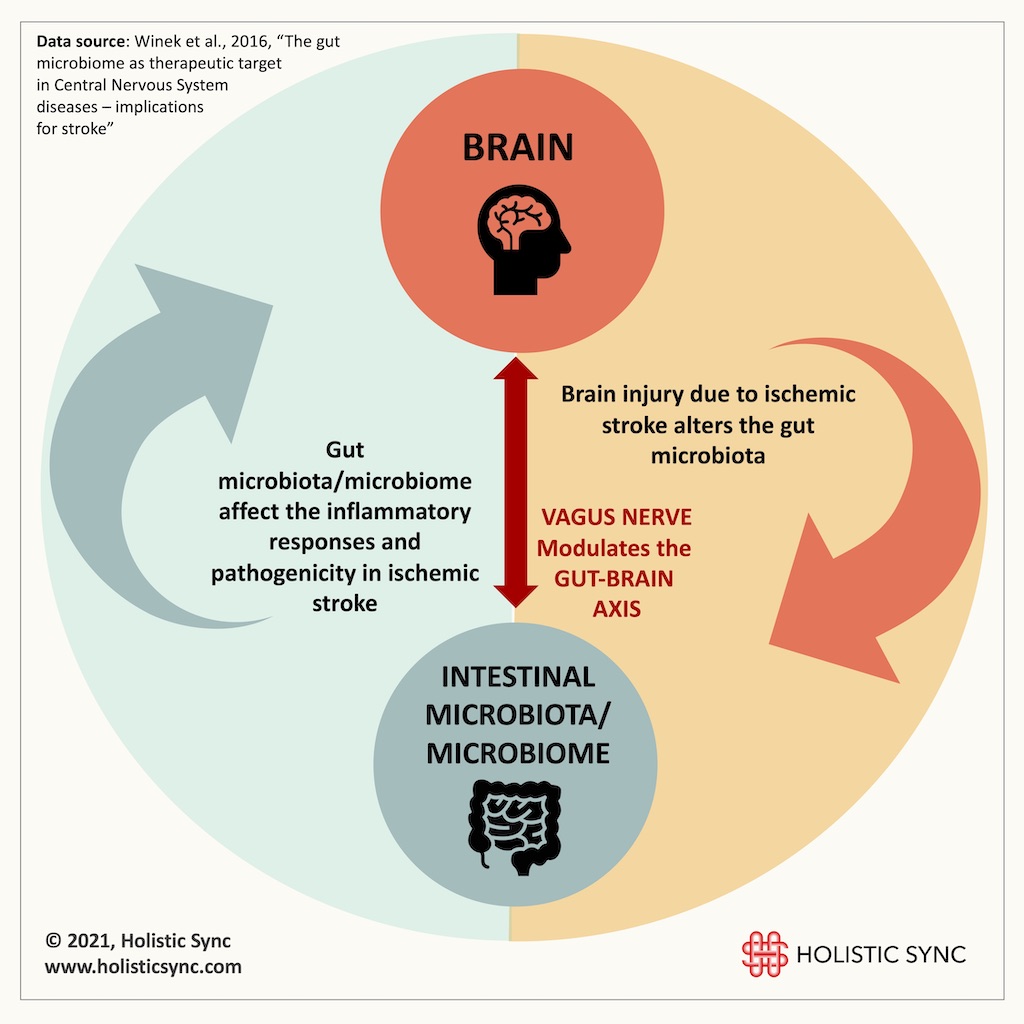
The microbiota is the collection of bacteria that populate the intestines and the microbiome is the collective microorganism, genome, metabolites, and gut environment.6 The microbiota contributes to several processes in the body such as providing vitamins, extracting nutritional components, and regulating neurotransmitters. The microbiota also fortifies the intestinal barrier, provides protection against pathogens, and contributes to the development of the immune, enteric, and central nervous systems.6 There is evidence that after stroke, the gut microbiota is modified leading to intestinal dysbiosis which induces gut inflammation.5,6 Dysbiosis refers to a change in the microbial composition commonly showing an increase in pathogenic organisms and a decrease in beneficial microbes. Changes in gut microbiota have important consequences for the immune system and also for the brain. Studies show that the gut microbiota regulates T cell homeostasis, and that T cells migrate from the intestines to the post-stroke brain. Thus, therapeutic measures that balance the gut microbiota and resolve dysbiosis may have beneficial effects during ischemic stroke.3
Despite some experimental studies showing that antibiotic therapies can be beneficial to address microbiota organisms related to certain diseases, broad-spectrum antibiotics often used post-stroke may deplete the good microbes, thus aggravating symptoms and making recovery more difficult.2 The studies point to a bidirectional relationship where brain injury alters gut microbiota but microbiota also affects the inflammatory responses in the ischemic brain.5 Also, the vagus nerve which is part of the parasympathetic nervous system (NS) provides an interface for the gut-brain axis. The gut microbiota modulates vagal nerve response, but the brain also influences the gut through the vagus nerve. Thus, the gut microbiota/microbiome and intestinal neuro-immunological changes should be considered when treating post-stroke symptoms and sequelae.
Post-stroke chronic inflammation
One important question for people suffering from stroke sequelae is regarding the post-stroke long-term impact of inflammation. How long can neuroinflammation and systemic inflammation persist after strokes? In recent years, researchers have been reporting that post-stroke neuroinflammation may last for months or years. The evidence shows that immune cells and cytokines that participate in an inflammatory process such as microglia and tumor necrosis-a (TNF-a) are chronically activated in areas of the brain often expanding to locations away from the original focal injury.7,8 The inflammatory responses in the injured brain are not limited to the site of damage but happen in the entire brain and persist for a long time.9 For instance, inflammatory cells can remain activated in patients with traumatic brain injury (TBI) 16-17 years after the acute injury.10,11 The consequences of this long-term injury to the brain may manifest as motor-sensory problems, depression, fatigue, cognitive impairment, and other problems.
In conclusion, there is a lot of evidence linking pre- and post-systemic inflammation to strokes. Chronic diseases that cause systemic inflammation present risks to stroke development and systemic inflammation aggravates neuroinflammation. Neuroinflammation and systemic inflammation can last a long time, even years after the acute stroke. Thus, evaluating pre- and post-stroke health status and addressing inflammation is essential to treating long-term symptoms and sequelae. Also, systemic post-stroke inflammation can overlap with other inflammatory conditions or diseases and make things more complex. Commonly, patients who experience strokes have a neuro-immunological response that goes beyond the brain such as liver, heart, spleen, bone marrow, adipose tissue, microbiota/microbiome, and the overall intestinal function.4,6 Apart from the central nervous system, a stroke involves the immune, endocrine, circulatory, and peripheral nervous systems. All these systems are interdependent and interconnected to each other. A holistic perspective of the whole body’s involvement is essential to devising treatment plans. Although ischemic stroke is the main subject of this article, hemorrhagic strokes present similar mechanisms.

Dr. Andréa Fuzimoto, DAOM, MSTCM, MSCS, CSAS, Dipl. O.M (NCCAOM®/USA), L.Ac. (CA/USA); PT/Acu (BR) is a clinician and researcher working with Holistic Integrative Medicine (HIM) with emphasis in gastrointestinal, neurological, and immunological conditions. Patients look for her careful diagnostic evaluation, strategic treatment planning, and compassionate care. As a researcher, she is a peer-reviewed published author and a Elsevier certified peer-reviewer contributing to different scientific journals. She has trained and worked in centers of excellence such as the Stanford University Medical School (Pain Medicine Division) with NIH-funded Clinical Trials, and the California Pacific Medical Center (CPMC), at the Stroke Clinic, among others. For more information on her specialties and certifications, visit Linkedin.
References
- Dziedzic T. Systemic inflammation as a therapeutic target in acute ischemic stroke. Expert Rev Neurother. 2015;(15(5):523-31). doi:https://doi.org/10.1586/14737175.2015.1035712
- Becker K, Buckwalter M. Stroke, Inflammation and the Immune Response: Dawn of a New Era. Neurotherapeutics. 2016;(13(4):659-660). doi:10.1007/s13311-016-0478-7
- Khoshnam S, Winlow W, Farzaneh M, Farbood Y, Moghaddam F. Pathogenic mechanisms following ischemic stroke. Neurol Sci. 2017;(38(7):1167-1186). doi:https://doi.org/10.1007/s10072-017-2938-1
- Dorrance A, Fink G. Effects of Stroke on the Autonomic Nervous System. Compr Physiol. 2015;(5(3):1241-63). doi:https://doi.org/10.1002/cphy.c140016
- Anrather J, Iadecola C. Inflammation and Stroke: An Overview. Neurotherapeutics. 2016;(13(4):661-670). doi:https://doi.org/10.1007/s13311-016-0483-x
- Winek K, Dirnagl U, Meisel A. The Gut Microbiome as Therapeutic Target in Central Nervous System Diseases: Implications for Stroke. Neurotherapeutics. 2016;(13(4):762-774). doi:https://doi.org/10.1007/s13311-016-0475-x
- Pappata S, Levasseur M, Gunn R, et al. Thalamic microglial activation in ischemic stroke detected in vivo by PET and [11C]PK1195. Neurology. 2000;(55(7):1052-4). doi:https://doi.org/10.1212/wnl.55.7.1052
- Gerhard A, Schwarz J, Myers R, Wise R, Banati R. Evolution of microglial activation in patients after ischemic stroke: a [11C](R)-PK11195 PET study. Neuroimage. 2005;(24(2):591-5). doi:https://doi.org/10.1016/j.neuroimage.2004.09.034
- Shi K, Tian D, Li Z, Ducruet A, MT Lawton, Shi F. Global brain inflammation in stroke. Lancet Neurol. Published online November 2019. doi:https://doi.org/10.1016/S1474-4422(19)30078-X
- Folkersma H, Boellaard R, Yaqub M, et al. Widespread and Prolonged Increase in (R)-11C-PK11195 Binding After Traumatic Brain Injury. J Nucl Med. 2011;(52(8):1235-9). doi:https://doi.org/10.2967/jnumed.110.084061
- Johnson V, Stewart J, Begbie F, Trojanowski J, Smith D, Stewart W. Inflammation and white matter degeneration persist for years after a single traumatic brain injury. Brain. 2013;(136(Pt1):28-42). doi:https://doi.org/10.1093/brain/aws322
