- Microbiota, Microbiome, Leaky Gut, Leaky Brain, Small Intestinal Bacterial Overgrowth (SIBO), Neurodegenerative Diseases, Alzheimer’s Disease, Parkinson’s Disease, Amyotrophic Lateral Sclerosis, Huntington’s Disease.
The microbiota-gut-brain axis and the central role of the gastrointestinal system in neurodegenerative diseases.

Author: Dr. Andréa Fuzimoto
Disclosure: This site contains links to our stores. Some others may be affiliate links by which we may earn a small commission when you purchase certain products or services. This does not affect the prices that you may pay for them. By purchasing from us you help support this website and our services. Thank you!
The research on intestinal microbiota caused a revolution in the understanding of neurodevelopmental, neurodegenerative, autoimmune, and chronic diseases, in general. We now know that gut microbes and their metabolites exert an essential influence in the maturation and development of the gastrointestinal, neurological, and immune systems, and the function of organ systems during one’s life. These structures evolve into an interconnected system which is designated the microbiota-gut-brain axis.
Have you ever wondered how our gastrointestinal, neurological, and immune systems evolved and matured? Many people have probably not given much thought to it… but there is something very surprising about it… When we are born, we receive the first microbial colonization. If you were vaginally delivered, you received the first microbial organisms (microbiota) from your mothers’ vaginal and fecal microbiota.1 However, if you were delivered through a c-section, you received a different set of microbes from your mothers’ skin and the hospital environment.1 Apart from these microbes, we acquire different ones depending on our dietary habits until the adult-like microbiota is developed by the age of 2 or 3 years old.2,1 So, each one of us develop a “unique” microbial profile that will impact organs systems and our health throughout our lives.
Research shows that the intestinal microbiota is essential for the maturation of the central nervous system (CNS), the intestinal or enteric nervous system (ENS), and immune system. Therefore, depending on the individual microbial profile, it influences how these systems will develop. One person may grow to be healthy, and another may have a set of specific microbes that influence the maturation of these systems in a way that predisposes that person to diseases. Researchers are studying if microbial signatures involving commensal and pathogenic microorganisms can help diagnose diseases. Regardless, the three systems are interconnected and the gut is a big influencer in neurological and immune diseases. Here, we look into some factors that can affect the gastrointestinal system and have an impact on neurodegenerative diseases through the microbiome-gut-brain axis.
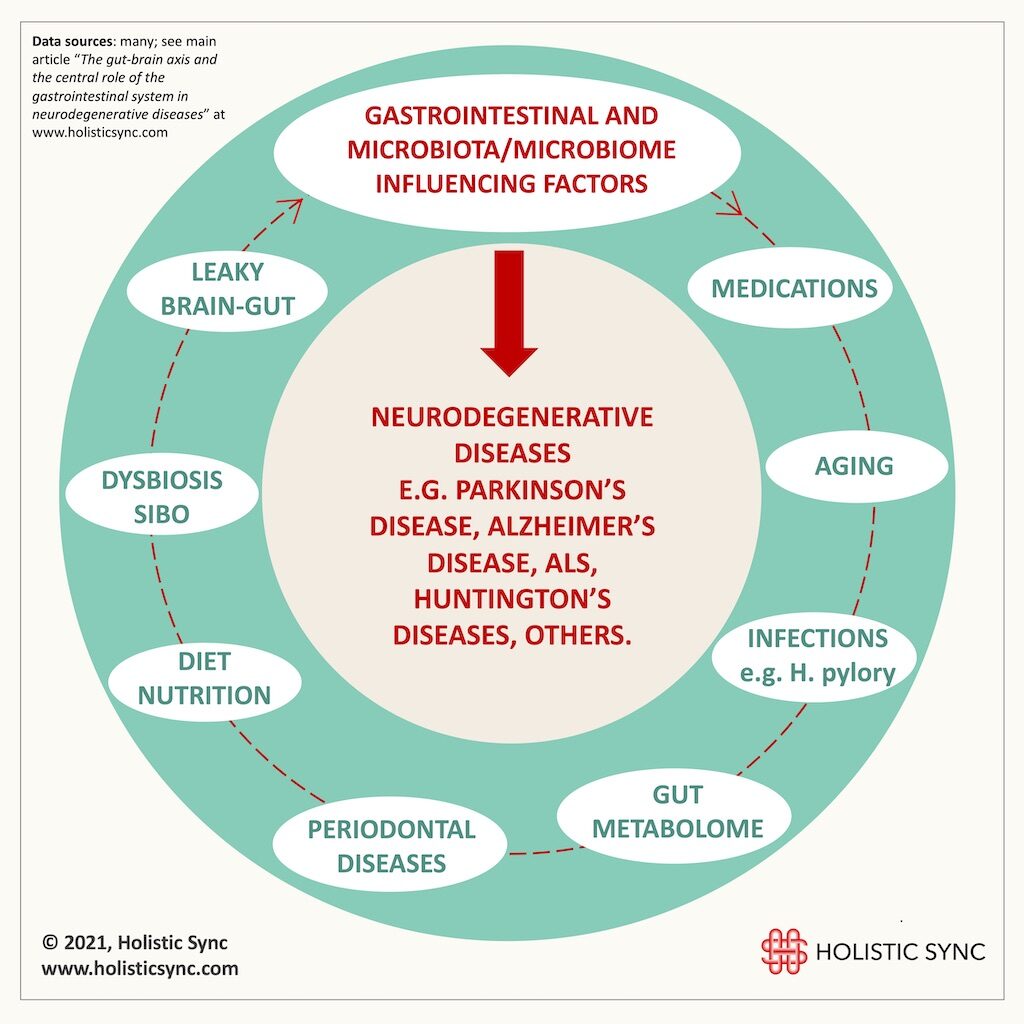
Gastrointestinal influencing factors in neurodegenerative diseases
1. Leaky Gut and Leaky Brain Connection
The intestinal epithelial lining provides a barrier that prevents unwanted substances from crossing from the gut lumen into the bloodstream. A disrupted gut barrier (leaky gut) may allow the passage of toxins, immune cells, pathogens, and big food particles into the blood circulation causing the production of inflammatory mediators that lead to systemic inflammation. Then, this inflammation may also disrupt the blood-brain-barrier (BBB) allowing undesirable substances to transmigrate into the brain causing neuroinflammation, neural injury, and degeneration (leaky brain).3 This is one of the pathways that would explain how microbiota changes and gut dysfunction can predispose and/or trigger neurodegenerative diseases.
Some studies demonstrated this pathological process in which the gut microbiota alters the intestinal and brain barriers contributing to the deposition of β-amyloid peptides that form plaques in the brain of Alzheimer’s disease patients.3,4 In a systematic review analyzing 47 papers (31 animal studies and 16 human studies) on the role of the gut microbiota in Alzheimer disease-derived dementia, the researchers concluded that the cognitive symptoms are regulated by the gut-brain axis.5 One recent article proposed the origin of Parkinson’s disease (PD) in the enteric nervous system (ENS).6 Leaky gut and an unbalanced microbiota (dysbiosis) may be the initial phase of PD when there are not even any motor symptoms present. These changes are accompanied by the activation of specific cells that amplify the inflammation, the enteric glial cells (EGCs), and the propagation of toxin proteins that clump in the brain of PD patients and that go from the ENS to the CNS, the α-synuclein proteins.6 When the first motor signs of PD appear, that means that there is already advanced damage to the dopaminergic neurons in the brain.6 Thus, the theory in which the gut is the source of PD would explain the presence of gastrointestinal (GI) dysfunctions in 30% of the patients preceding motor symptoms and which exacerbate only in a later stage of the disease.6 Some of these gut manifestations include dyspepsia, hypersalivation from reduced swallowing, constipation, nausea, and abdominal pain.7
Motor neuron death is the hallmark of Amyotrophic Lateral Sclerosis (ALS).8 In an investigation using an animal model of ALS, the mice showed leaky gut, impaired microbiome, an increase in inflammatory cytokines, and abnormal Paneth cells before the onset of ALS neuromuscular symptoms.8 Therefore, loss of barrier function and dysbiosis may also be an early stage of ALS. Huntington’s disease (HD) which is characterized by motor, cognitive and psychiatric problems is also suspected of being associated with intestinal pathological dysfunctions. In an animal model of HD, the mice also presented leaky gut and dysbiosis.9 It is certainly no coincidence that weight loss, food malabsorption, diarrhea, and gut dysmotility are important non-neurological complications found in HD mice models, and also commonly present in HD patients. Weight loss was correlated with disease progression, and the protein associated with HD, huntingtin, is produced in the brain as well as in the gastrointestinal tract (GIT).10 Likewise, people with multiple sclerosis (MS) showed changes in the microbiome, increased intestinal permeability, and altered bile acid metabolism.11 Although a lot more research is necessary, the emerging evidence points to the central role of the gastrointestinal system in neurodegenerative diseases.
2. Helicobacter pylori
H. pylori is a bacteria that can infect the digestive tract and that can cause gastritis, stomach ulcers, and in some cases stomach cancer. A meta-analysis research with 33,125 participants concluded that H. pylori infection might be associated with PD.15 Some hypotheses connect H. pylori with Parkinson’s disease:
- Inflammation: The chronic inflammation caused by H. pylori can expand into a systemic inflammation, and cells derived from this immune response could cross the blood-brain-barrier (BBB) and get into the brain, stimulate microglial activation, and trigger and/or exacerbate the neuroinflammation seen in PD.10 This is a plausible theory as there is accumulating evidence that leaky brain is involved in the pathogenesis of PD.10 However, more research is necessary to investigate this theory.
- Microbiota: In this hypothesis, the H. pylori infection would cause a microbiota dysregulation affecting the gut metabolism and the neuro-endocrine-immune response. Thus, this could cause inflammation and disrupt the gut-brain axis and lead to PD.10 In fact, one research analyzed the gut microbial composition in PD patients and found out that PD is accompanied by dysbiosis with a potential microbial signature.16 In the future, scientists may be able to identify PD through a microbiota-microbiome signature years before disease development and elaborate treatment options.
- Brain Infection: This theory predicts that the bacteria could hide inside some immune cells, transit through the circulation, and cross the BBB to enter into the brain. So far, there is no proof for this hypothesis.10
- Toxins: H. pylori produce several toxins, and these toxins could disrupt and cross the blood-brain barrier (BBB) and cause neurotoxicity. However, PD-associated-toxins have not been assessed through research to determine their impact on the disease.10
Regardless, the existing scientific studies show that people with PD are 1.5-3 fold more likely to be infected with H. pylori than the uninfected people, and those infected have worse motor functions than those not harboring the bacteria.10 Also, eradication of the H. pylori improved the motor function of PD patients compared with those that did not eliminate it and improved levodopa absorption, the medication often used for PD.10 More investigations will be necessary to clarify the mechanisms involved in H. pylori infection and PD. Interestingly, H. pylori may also impact the development and Alzheimer’s disease through different mechanisms.17 H. pylori alters the pH of the stomach and consequently changes the microbiota of both the stomach and gut. Thus, H. pylori can modify the balance of the whole gastrointestinal system, and in this sense, it could be associated with different extra gastric diseases including neurodegenerative conditions.
3. Small Intestinal Bacterial Overgrowth (SIBO)
SIBO is characterized by an excessive amount of bacteria in the small intestine. Normally, the small intestine has a low diversity and density of bacteria. However, with the SIBO, microbes from other parts of the gastrointestinal system colonize the small intestine causing dysbiosis and symptoms such as bloating, abdominal pain, diarrhea, constipation, nausea, poor appetite, and fatigue. Remarkably, SIBO has been associated with Parkinson’s disease (PD). One paper reported a high prevalence of SIBO in PD patients when compared with the controls (54.17% vs. 8.33%, respectively).15 In another study evaluating SIBO in 103 PD patients, 25.3% of these patients were SIBO-positive and it predicted worse motor function for them.16 Also, a case-control study examined the presence of SIBO in multiple sclerosis (MS) patients. They found a high prevalence of SIBO in the MS patients when compared to control subjects (38.14% vs 8.47%, respectively).17 These results do not establish SIBO as the cause of these diseases. Instead, they show that there is an imbalance of the microbial communities in the intestines and that it could be one of the factors contributing to developing, triggering, or worsening neurodegenerative diseases.
4. Periodontal (gum) diseases
During the past years, several studies suggested the association between poor health of the mouth-gum with neurodegenerative diseases such as Alzheimer’s disease (AD) and dementia. A systematic review article with meta-analysis found a significant correlation between periodontal disease and AD.18 Periodontitis is a chronic inflammatory disease that destroys the gingival connective tissue, cementum, and alveolar bone caused by bacterial biofilm formed by pathogens such as Porphyromonas gingivalis, Treponema denticola, and Tannerella forsythia19. These microbes can trick the host immune system and create a toxic environment to grow and survive, and sustain an inflammatory environment.19 These periodontal pathogens may lead to intestinal dysbiosis, chronic systemic inflammation, and/or cross into the central nervous system (CNS), as it is suspected with P. gingivalis.19
In animal experiments of Alzheimer’s disease, P. gingivalis transmigrated into the brain, caused peripheral and intracerebral inflammation, and impaired vascular and microvascular integrity.20 In another study, patients with periodontal inflammatory disease showed a significantly higher risk to develop Parkinson’s disease (PD).21 The causal relationship between periodontal diseases and neurodegenerative diseases is uncertain. However, caries and gum disease are notably known to cause oral-local as well as systemic problems. Poor oral health is increasingly being linked to AD, rheumatoid arthritis, atherosclerosis, diabetes mellitus, stroke, myocardial infarction, and many other diseases.22 Since the mouth is the “door” to the gastrointestinal system and pathogens harbored there can travel to other parts of the body, this is also a focal point for health care in neurodegenerative diseases.
5. Medications and early-life antibiotic use
Medications such as antibiotics, proton pump inhibitors, metformin, and other over-the-counter drugs alter the gut microbiota.3 Studies show that the use of antibiotic drugs early in life considerably impacts the gut microbiota and predispose to the development of inflammatory and metabolic diseases later in life.3 The research on germ-free animals is important for neurodegenerative diseases because it shows how health is impacted when there are no microbes to assist the body’s functions. Investigations on Alzheimer’s disease (AD) utilizing germ-free mice showed a reduction of β-amyloid deposition in the brain, thus suggesting the role of microbes in the pathogenesis of AD.3 In one research, a long-term broad-spectrum combinatorial antibiotic therapy decreased the β-amyloid plaques in an animal model of AD.23 In another animal model of Parkinson’s disease (PD), the administration of non-absorbable antibiotics attenuated dopaminergic cell damage and motor impairment.24 So, antibiotics can be both detrimental, if tampering with the right composition of the microbiota, or beneficial if eradicating pathogens possibly related to the pathogenesis of neurodegenerative diseases. Either way, the research done so far supports an association between gut microbes and some neurodegenerative diseases.
6. Diet and Nutrition
Foods and drinks influence the composition of the gut microbiota, and alterations of the microbiota are related to chronic diseases, including obesity and inflammatory conditions.25 The prevalence of certain dietary components such as carbohydrates, proteins, fats fibers, vitamins, processed foods, or vegetable/fruit-based nutrition modify the intestinal microbial milieu and change the response of the immune system.3 In a study with 178 elderly people, the researchers collected fecal microbiota of residents of different dwellings (community, out-of-hospital, short-term rehabilitation, long-term residential care units, etc.) and studied their impact on the deterioration of their immune system and frailty status.25 They concluded that the diet of people living in the same location determined a similar microbial composition and metabolites, and a diversified diet promoted more diverse gut microbiota.25 The healthiest people ate a more varied diet which was different from those in long-term residential care who showed increased frailty, inflammatory markers (TNF-α, IL-6, IL-8, and C-reactive protein), and reduced muscle mass.25 They also scored poorly in measures of geriatric depression, functional independence, and mental state.25 Thus, what each group ate made a huge difference in their health, and the authors defended the use of nutrition to modulate the microbiota toward a less inflammatory diet.
The consumption of ultra-processed foods and simple sugars with a significant decrease in fibers and vegetables causes dysregulation of the microbiota-microbiome, generates a low-grade inflammation, increases inflammatory cytokines and oxidative biomarkers, and favors cognitive impairment and neurodegenerative diseases.26 Currently, studies show that the synergistic action of combined dietary approaches and supplements that provide anti-inflammatory resources offer the strongest approach to benefit Alzheimer’s diseases (AD) and dementia patients.27 Increasing evidence also suggests that a nutrition-rich in fibers, bioflavonoids, and fatty acids modulate the microbiota-microbiome, intestinal and brain barriers, and reduce inflammatory markers thus benefiting patients with PD.28 Other approaches such as intermittent fasting and a ketogenic diet may also provide similar benefits for these patients.28 The study of the gut microbial constitution and nutrition is reinforcing the notion that food is medicine, and that it can be used to treat diseases and improve our health.
7. Aging
The microbiota is a regulator of brain development, aging, and neurodegeneration.29 Thus, the microbes in our body influence all phases of development and also the decline into old age. At the same time, aging causes changes in microbiota, gut barrier, blood-brain barrier (BBB), and immune system.3 Aging promotes a decrease in the diversity of the microbiota-microbiome, and a healthy aging process correlates with a more diverse microbiome composition.29 Given the growing research on the subject, it is very much possible that manipulation of the microbiota and modulation of the immune system could, someday, lead to effective therapeutic approaches to curb neurodegenerative diseases but also the aging process.
8. Gut Metabolome
The gut metabolome refers to the metabolic substances produced by the microbial communities. Scientists are also studying how these metabolites may contribute to diseases. For example, in one research with 64 Parkinson’s disease (PD) patients and 51 controls, there was a significant decrease in certain microbes that are liked to anti-inflammatory and neuroprotective activity but also changes in metabolites revealing the presence of bacteria of the Lachnospiraceae family.30 Alterations in microbiota-producing metabolites have been associated with neurodegenerative and neuropsychiatric diseases (e.g. Alzheimer’s disease, autism spectrum disorders, bipolar disorder, depression, Huntington’s disease, Parkinson’s diseases, post-traumatic stress disorder (PTSD), schizophrenia, and others).31 The study of the metabolites produced by the microbiota of patients in neurodegenerative diseases can provide valuable information on possible biomarkers of disease, detection of altered metabolic pathways and signatures, and also the identification of possible therapeutics. Furthermore, different diets alter the microbes’ metabolic responses in the gut and this is another way foods can act in preventing and healing some diseases.
Currently, neurodegenerative diseases are considered multi-factorial. Different factors may predispose, trigger, or worsen these diseases such as genetic susceptibility, environmental determinants, neuro-immune system status, age, comorbidities, nutritional habits, and many others that were not mentioned in this article. Although there is not enough evidence to establish a causal relationship between the gastrointestinal system and neurodegenerative diseases, there is certainly enough information showing that gastrointestinal function plays a key role in these conditions. As we saw, in many cases the GI symptoms precede the neurological symptoms, and this may happen years before the onset of neurodegenerative disease. The objective identification of deficiencies in the GI function may guide in correcting and normalizing the microbiota-microbiome/gut-brain axis and assist in the prevention and treatment of chronic diseases.

Dr. Andréa Fuzimoto, DAOM, MSTCM, MSCS, CSAS, Dipl. O.M (NCCAOM®/USA), L.Ac. (CA/USA); PT/Acu (BR) is a clinician and researcher working with Holistic Integrative Medicine (HIM) with emphasis in gastrointestinal, neurological, and immunological conditions. Patients look for her careful diagnostic evaluation, strategic treatment planning, and compassionate care. As a researcher, she is a peer-reviewed published author and a certified peer-reviewer contributing to different scientific journals. She has trained and worked in centers of excellence such as the Stanford University Medical School (Pain Medicine Division) with NIH-funded Clinical Trials, and the California Pacific Medical Center (CPMC), at the Stroke Clinic, among others. For more information on her specialties and certifications, visit Linkedin.
References
- Births Ans Natality. Center for Disease Control and Prevention (CDC) https://www.cdc.gov/nchs/fastats/births.htm
- Forssberg H. Microbiome programming of brain development: implications for neurodevelopmental disorders. Dev Med Child Neurol. 2019;(61(7):744-749). doi:https://doi.org/10.1111/dmcn.14208
- Addis M, Tanca A, Uzzau S, Oikonomou G, Bicalho R, Moroni P. The bovine milk microbiota: insights and perspectives from -omics studies. Mol Biosyst. 2016;(12(8):2359-72). doi:https://doi.org/10.1039/c6mb00217j
- Berg G, Rybakova D, Fischer D, et al. Microbiome definition re-visited: old concepts and new challenges. Microbiome. Published online June 30, 2020. doi:https://dx.doi.org/10.1186%2Fs40168-020-00875-0
- Quigley E. Microbiota-Brain-Gut Axis and Neurodegenerative Diseases. Curr Neurol Neurosci Rep. 2017;(17(12):94). doi:https://doi.org/10.1007/s11910-017-0802-6
- Endres K, Schafer K. Influence of Commensal Microbiota on the Enteric Nervous System and Its Role in Neurodegenerative Diseases. J Innate Immun. 2018;(10(3):172-180). doi:https://dx.doi.org/10.1159%2F000488629
- Kohler O, Krogh J, Mors O, Benros M. Inflammation in Depression and the Potential for Anti-Inflammatory Treatment. Curr Neuropharmacol. 2016;(14(7):732-42). doi:https://doi.org/10.2174/1570159×14666151208113700
- Ticinesi A, Tana C, Nouvenne A, Prati B, Lauretani F, Meschi T. Gut microbiota, cognitive frailty and dementia in older individuals: a systematic review. Clin Interv Aging. 2018;(13:1497-1511). doi:https://dx.doi.org/10.2147%2FCIA.S139163
- Seguella L, Sarnelli G, Esposito G. Leaky gut, dysbiosis, and enteric glia activation: the trilogy behind the intestinal origin of Parkinson’s disease. Neural Regen Res. 2020;(15(6):1037-1038). doi:https://doi.org/10.4103/1673-5374.270308
- McGee D, Lu X, Disbrow E. Stomaching the Possibility of a Pathogenic Role for Helicobacter pylori in Parkinson’s Disease. J Park Dis. 2018;(8(3):367-374). doi:https://dx.doi.org/10.3233%2FJPD-181327
- Wu S, Yi J, Zhang Y, Zhou J, Sun J. Leaky intestine and impaired microbiome in an amyotrophic lateral sclerosis mouse model. Physiol Rep. 2015;(3(4):e12356). doi:https://doi.org/10.14814/phy2.12356
- Stan T, Soylu-Kucharz R, Burleigh S, et al. Increased intestinal permeability and gut dysbiosis in the R6/2 mouse model of Huntington’s disease. Sci Rep. 2020;(10(1):18270). doi:https://doi.org/10.1038/s41598-020-75229-9
- Vanderburg J, Winqvist A, Aziz N, et al. Gastrointestinal dysfunction contributes to weight loss in Huntington’s disease mice. Neurobiol Dis. 2011;(44(1):1-8). doi:https://doi.org/10.1016/j.nbd.2011.05.006
- Camara-Lemarroy C, Metz L, Yong V. Focus on the gut-brain axis: multiple sclerosis, the intestinal barrier and the microbiome. World J Gastroenterol. 2018;(24(37): 4217-4223). doi:https://doi.org/10.3748/wjg.v24.i37.4217
- Shen X, Yang H, Wu Y, Zhang D, Jiang H. Meta-analysis: Association of Helicobacter pylori infection with Parkinson’s diseases. Helicobacter. 2017;(22(5)). doi:https://doi.org/10.1111/hel.12398
- Hill-Burns E, Debelius J, Morton J, et al. Parkinson’s disease and Parkinson’s disease medications have distinct signatures of the gut microbiome. Mov Disord. 2017;(32(5):739-749). doi:https://doi.org/10.1002/mds.26942
- Franceschi F, Ojetti V, Candelli M, et al. Microbes and Alzheimer’ disease: lessons from H. pylori and GUT microbiota. Eur Rev Med Pharmacol Sci. 2019;(23(1):426-430). doi:https://doi.org/10.26355/eurrev_201901_16791
- Gabrielli M, Bonazzi P, Scarpellini E, et al. Prevalence of small intestinal bacterial overgrowth in Parkinson’s disease. Mov Disord. 2011;(26(5):889-92). doi:https://doi.org/10.1002/mds.23566
- Fu P, Gao M, Yung K. Association of Intestinal Disorders with Parkinson’s Disease and Alzheimer’s Disease: A Systematic Review and Meta-Analysis. ACS Chem Neurosci. 2020;(11(3):395-405). doi:https://doi.org/10.1021/acschemneuro.9b00607
- Zhang Y, Liu G, Duan Y, Han X, Dong H, Geng J. Prevalence of Small Intestinal Bacterial Overgrowth in Multiple Sclerosis: a Case-Control Study from China. J Neuroimmunol. 2016;(301:83-87). doi:https://doi.org/10.1016/j.jneuroim.2016.11.004
- Leira Y, Domínguez C, Seoane J, et al. Is Periodontal Disease Associated with Alzheimer’s Disease? A Systematic Review with Meta-Analysis. Neuroepidemiology. Published online 2017. doi:https://doi.org/10.1159/000458411
- Singhrao S, Harding A, Poole S, Kesavalu L, Crean S. Porphyromonas gingivalis Periodontal Infection and Its Putative Links with Alzheimer’s Disease. Mediat Inflamm. 2015;(2015:137357). doi:https://doi.org/10.1155/2015/137357
- Harding A, Robinson S, Crean S, Singhrao S. Can Better Management of Periodontal Disease Delay the Onset and Progression of Alzheimer’s Disease? J Alzheimers Dis. 2017;(58(2):337-348). doi:https://doi.org/10.3233/jad-170046
- Chen C, Wu Y, Chang Y. Periodontal inflammatory disease is associated with the risk of Parkinson’s disease: a population-based retrospective matched-cohort study. Peer J. 2017;(5:e3647). doi:https://doi.org/10.7717/peerj.3647
- Scannapieco F, Cantos A. Oral inflammation and infection, and chronic medical diseases: implications for the elderly. Periodontol 2000. 2016;(72(1):143-75). doi:https://doi.org/10.1111/prd.12129
- Minter M, Zhang C, Leone V, et al. Antibiotic-induced perturbations in gut microbial diversity influences neuro-inflammation and amyloidosis in a murine model of Alzheimer’s disease. Sci Rep. 2016;(6:30028). doi:https://doi.org/10.1038/srep30028
- Koutzoumis D, Vergara M, Pino J, et al. Alterations of the gut microbiota with antibiotics protects dopamine neuron loss and improve motor deficits in a pharmacological rodent model of Parkinson’s disease. Exp Neurol. 2020;(325:113159). doi:https://doi.org/10.1016/j.expneurol.2019.113159
- Cleasson M, Jeffrey I, Conde S, et al. Gut microbiota composition correlates with dietand health in the elderly. Nature. 2012;(488(7410):178-84). doi:https://doi.org/10.1038/nature11319
- Leo E, Campos M. Effect of ultra-processed diet on gut microbiota and thus its role in neurodegenerative diseases. Nutrition. 2020;(71:110609). doi:https://doi.org/10.1016/j.nut.2019.110609
- Szczechowiak K, Diniz B, Leszek J. Diet and Alzheimer’s dementia – Nutritional approach to modulate inflammation. Pharmacol Biochem Behav. 2019;(184:172743). doi:https://doi.org/10.1016/j.pbb.2019.172743
- Jackson A, Frosyth C, Shaikh M, et al. Diet in Parkinson’s Disease: Critical Role for the Microbiome. Front Neurol. 2019;(10:1245). doi:https://doi.org/10.3389/fneur.2019.01245
- Dinan T, Cryan J. Gut instincts: microbiota as a key regulator of brain development, ageing and neurodegeneration. J Physiol. 2017;(595(2):489-503). doi:https://doi.org/10.1113/jp273106
- Vascellari S, Palmas V, Melis M, et al. Gut Microbiota and Metabolome Alterations Associated with Parkinson’s Disease. nSystems. 2020;(5(5):e00561-20). doi:https://doi.org/10.1128/msystems.00561-20
- Konjevod M, Perkovic M, Sáiz J, Strac D, Barbas C, Rojo D. Metabolomics analysis of microbiota-gut-brain axis in neurodegenerative and psychiatric diseases. J Pharm Biomed Anal. 2021;(194:113681). doi:https://doi.org/10.1016/j.jpba.2020.113681
